Sign Tracking Predicts Increased Chouce of Cocaine Over Food in Rat
Chapter 6: Sign-Tracking, Response Inhibition, and Drug-Induced Vocalizations
Behavioral Neuroscience Program, Department of Psychology, University at Buffalo
Corresponding Author:
Paul J. Meyer, PhD, B72 Park Hall, University at Buffalo, Buffalo NY 14051; e-mail:pmeyer@buffalo.edu ; phone: (716) 645-0263; fax: (716) 645-3651.
Abstract
Individual variation in sign- and goal-tracking during Pavlovian conditioned approach (PavCA) is thought to reflect a psychological trait that we refer to as the tendency to attribute incentive value (incentive salience) to reward cues. But is incentive salience attribution truly a personality trait that reflects temperament, like impulsivity or sensation seeking? An alternative explanation is that these subgroups of rats differ in general learning processes. For example, the initial value placed on the unconditioned stimulus (US) may explain the differences in the development of sign- and goal-tracking and some of the correlated traits associated with these behaviors. However, while many findings can be explained by such differences in general learning processes, some, such as cocaine-induced vocalizations, cannot. In this chapter we review examples of behaviors in other paradigms that are associated with sign-tracking and goal-tracking, focusing on (1) the deficits in response inhibition and enhanced impulsivity that are apparent in sign-trackers, and (2) the seemingly disparate association of sign-tracking with drug-induced vocalizations. In integrating these findings, we suggest that sign-tracking is associated with poor inhibitory control that leads to increased impulsivity and enhanced responsivity to conditioned stimuli (CSs)[1] associated with rewards. Further, once this learning has occurred, CSs can have enduring influences upon behavior that depend on their acquired incentive properties, but that can also be updated as the value of the US changes.
Keywords: attention; autoshaping; cocaine; devaluation; goal-tracking; impulsivity; incentive salience; omission; sensitization; sign-tracking; suboptimal choice; ultrasonic vocalizations
Introduction
A classic study by Breland and Breland (1961), while not explicitly measuring sign-tracking, showed abnormal attachment to food-associated stimuli. When they trained raccoons to deposit coins into a receptacle to earn food, they reported that "Not only could [the raccoon] not let go of the coins, but he spent seconds, even minutes, rubbing them together (in a most miserly fashion), and dipping them into the container. He carried on this behavior to such an extent that the practical application we had in mind—a display featuring a raccoon putting money in a piggy bank—simply was not feasible. The rubbing behavior became worse and worse as time went on, in spite of nonreinforcement." This may be better described as an extreme example of conditioned reinforcement, because these raccoons were trained in an operant and not a Pavlovian paradigm. In fact, as discussed later in the text, studies with sign- and goal-trackers suggest that the persistent behavior that occurs during instrumental conditioning is different than that which occurs during Pavlovian conditioning. However, it is an early demonstration of how excessive attribution of incentive salience to a reward cue can lead to maladaptive behavior.
In her 1982 review, Karen Hollis describes her "prefiguring" hypothesis, in which sign-tracking is an adaptive response in that it enables "the animal to optimize interaction with the forthcoming biologically important event (US) [which] allows the animal to better deal with the US event, and as such, the [conditioned response] is essentially preparatory" (Hollis 1982). Hollis says that it is irrelevant to her functional argument that the CS and US are separated in the laboratory, essentially because this separation rarely occurs in the real world. Yet, these laboratory experiments demonstrate that sign-tracking is a poorly controlled conditioned response and is related to poor response inhibition in animal models of impulsivity (Tomie 1996). This has translational impact, because modern human environments frequently accomplish exactly what Hollis deemed irrelevant by separating the CS and US, and is a common factor among many disorders, including drug addiction, obsessive-compulsive disorder, and other cue-controlled disorders such as problem gambling and post-traumatic stress disorder (Anselme this volume; Morrow this volume; Robinson et al. this volume; Tomie et al. this volume). For these disorders, the cues are often far-removed from the primary reinforcer (e.g., the drug or money). Thus, even though sign-tracking may have evolved as an adaptive behavior, understanding how sign-tracking can lead to maladaptive behavior in modern environments is a key goal from basic learning and translational perspectives. In the next two sections, we provide several examples of sign-tracking being a poorly controlled response, and how individuals predisposed to sign-track engage in other putatively maladaptive behaviors.[2]
Sign-Tracking despite Non-Reinforcement
Omission
Omission studies are particularly interesting because they impose a negative contingency on the subjects' behavior. Specifically, if the subject makes a sign-tracking response, the reward is not delivered. Adding this instrumental contingency would be expected to decrease sign-tracking rapidly, because in doing so the number of rewards would be maximized. Yet, several studies suggest that under omission conditions, sign-tracking is reduced only slowly and does not completely disappear (Herrnstein and Loveland 1972; Hollis 1982; Moore 1973; Williams and Williams 1969). This is particularly evident in studies with pigeons (Williams and Williams 1969) but can be seen in rat studies as well. For example, an early study with rats (Davey et al. 1981) found that, while lever pressing was reduced by omission, rats still approached and investigated the lever. This "nosing" behavior increased as a result of the omission contingency. In our own studies, we trained rats in a PavCA paradigm, and after five days of training, switched to an omission contingency in which either a sign-tracking or a goal-tracking response resulted in the non-delivery of the food pellet. As can be seen in Figure 6.1, lever-presses declined throughout training (as did goal-tracking, not shown in the figure). However, during this experiment we equipped the levers and their surrounding faceplates with contact detectors, which enable the detection of approach that does not result in a lever press. We found that there was no effect of omission on this measure of sign-tracking. Thus, as in Davey et al. (1981) and in other studies (Chang and Smith 2016; Locurto et al. 1976; Stiers and Silberberg 1974) we found that sign-tracking was still present, although the topography of the response had been altered. These findings suggest that sign-tracking is not completely independent of the US such that it cannot be modified, but also that the incentive properties of the CS remain intact despite the negative behavioral contingency.
Extinction
Subsequent studies using Pavlovian conditioning have demonstrated a similar phenomenon regarding poorly controlled sign-tracking in the face of non-reinforcement. In the extinction paradigm, the food reward is simply withheld while the CS continues to be presented. When this is done in a Pavlovian paradigm, the conditioned response (in our case sign-tracking and goal-tracking) decreases in frequency and eventually ceases. For example, Stringfield et al. (2018) removed the US (a sucrose solution) during a single extinction session and observed a reduction in goal-tracking, but not sign-tracking. In a study by Beckmann and Chow (2015), sign- and goal-tracking were conditioned to separate stimuli, and they showed that sign-tracking was more resistant to extinction as well. This 2-CS model is useful for determining learning mechanisms of conditioned approach behaviors, because it allows the simultaneous assessment of sign- and goal-tracking in the same individual. However, for this same reason, it is not ideal for studies targeted toward determining whether individual vulnerabilities render certain subjects resistant to extinction.
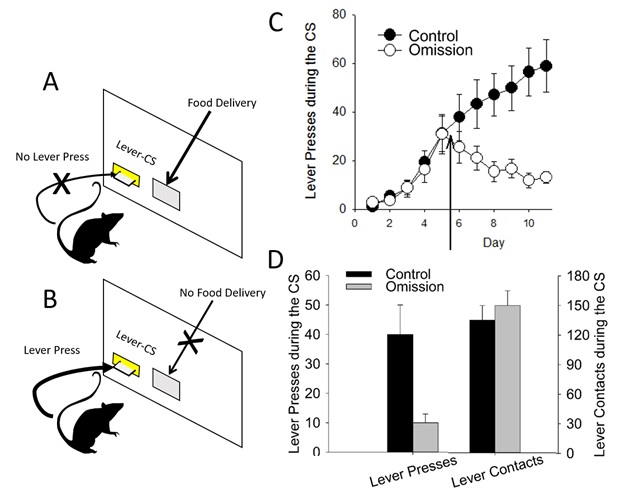
Figure 6.1: In omission training, a food pellet is delivered only if the subject does not press the lever-CS (panel A) but not if it does (panel B). This leads to a decrease in the number of lever presses (panel C) but not in the number of lever contacts (panel D). Arrow indicates that omission procedure was enacted after day 5 of testing.
Ahrens et al. (2016) compared extinction rates in sign- and goal-trackers and demonstrated that sign-tracking extinguishes more slowly than goal-tracking. However, there were no apparent differences between sign- and goal-trackers in the extinction of instrumental responses, for example, nose-poke responding for either food or cocaine (Ahrens et al. 2016; Saunders and Robinson 2011; Yager and Robinson 2010). This instrumental versus Pavlovian distinction is important for two reasons. First, instrumental extinction typically occurs in the absence of any cues, while the CS is present during Pavlovian extinction. Thus, the presence of the CS during Pavlovian extinction is the key distinguishing factor underlying sign- and goal-tracker differences in these two paradigms. Second, it suggests sign- and goal-trackers do not differ in this form of inhibitory learning generally, because only Pavlovian, and not instrumental, extinction is different between these two subgroups.
Outcome Devaluation
A third way to study the persistence of sign-tracking is through outcome devaluation. Instead of omitting the US, subjects can be US-satiated, that is, they are given a large quantity of the US before testing, thus reducing the relative value of the US. For example, a recent study in humans (De Tommaso et al. 2017) found that a water-paired CS biased attention in thirsty subjects even after they were allowed to drink. Alternatively, the US can be replaced with something less valued, aversive (e.g., quinine), or paired with an illness-inducing drug such as lithium chloride. This latter method is ideal, because the US illness pairing occurs in the absence of the CS, and thus changes in sign- and goal-tracking cannot be attributed to alterations of learning (Colwill and Motzkin 1994; Holland and Straub 1979). The idea is that any reduction in sign-tracking or goal-tracking observed after devaluation provides evidence that these responses are driven by the ability of the CS to evoke an internal representation of the US. Alternatively, if the devaluation does not cause a change in sign- or goal-tracking, that suggests the incentive value of the CS and its ability to evoke a response controls behavior independently of the US.
Morrison et al. (2015), after establishing sign- and goal-trackers in a group of rats, gave injections of lithium chloride after being allowed to consume the US (liquid sucrose) in the home cage for 20 minutes. They found that sign-tracking was enhanced after this treatment, while goal-tracking was reduced. Further, by examining sign-trackers and goal-trackers separately, they found that most of these changes were due to changes in goal-trackers, but not sign-trackers. A study by Nasser et al. (2015) showed a similar result using a slightly different procedure. They first paired a light stimulus with a food pellet (which elicited only goal-tracking in all rats), and then paired the food pellet with lithium chloride. The effectiveness of the devaluation was measured by examining the response to the light CS. Then, after this test, PavCA was conducted to determine which rats were sign- or goal-trackers. The authors found a positive correlation between the response to the light CS and the magnitude sign-tracking during PavCA. Like Morrison et al. (2015), this indicates that sign-trackers, relative to goal-trackers (Nasser grouped goal-trackers and intermediates together in this study), are relatively insensitive to the US devaluation. They also performed a second order conditioning task, in which a second stimulus was paired with their light CS, but there was no relationship between the response to this second stimulus and sign-tracking. However, this does raise an interesting experimental idea: would the lever CS from PavCA support greater second-order conditioning in sign-trackers versus goal-trackers? If so, this would provide further evidence that the CS supports conditioning independent of the US. While this study has not been done, one might predict this outcome based on the experiments showing that the CS serves as a better conditioned reinforcer in sign-trackers relative to goal-trackers (Meyer et al. 2014; Robinson and Flagel 2009).
However, other studies have found that US devaluation does alter the sign-tracking response. For example, Cleland and Davey (Cleland and Davey 1982; Davey and Cleland 1984) devalued the US in rats both by satiation and using lithium chloride. They found that sign-tracking and goal-tracking were reduced similarly by both of these methods. Interestingly, rats oriented more to the lever and the goal following devaluation. The authors argued that satiation had weakened a response chain consisting of orient-approach-contact, with the latter components of this chain preferentially weakened by satiation and thus making it more likely to observe the orientation component. Another study using lithium chloride devaluation (Derman and Delamater 2014) found a similar effect and also demonstrated that an already learned goal-tracking response inhibits the development of a sign-tracking response, and vice-versa.[3]
Increasing US Value
Together, these studies show that the sign-tracking response is influenced by the value of the US. Perhaps the strongest evidence of this includes experiments where increasing the value of the US increases the sign-tracking response (Robinson and Berridge 2013). In this experiment, pairing a concentrated salt solution with a lever CS evoked low levels of sign-tracking, but once the rats were tested in a salt-deprived state, rats approached the CS immediately, even before additional learning had occurred. Thus, changing the value of the US in this experiment had powerful and instantaneous effect on the incentive value of the CS. Davey and Cleland (1984) also showed that (1) giving rats food that was not paired with the CS or (2) presenting rats with a food-paired auditory CS both increased sign-tracking. This suggests that the enhanced probability of "extra" USs occurring in these two conditions, whether or not the extra USs were actually presented, enhanced the degree of sign-tracking.
Impulsivity
Given the evidence that CSs acquire incentive salience and lead to poorly controlled sign-tracking responses, several studies have examined whether sign-tracking is related to other maladaptive responses. For example, impulse-control disorders are characterized by failure to resist temptation or urges. Generally speaking, these disorders can be defined by poor inhibitory control and self-regulation. However, impulsivity can be further subdivided into two subcategories, including impulsive choice and impulsive action. Classic laboratory models of these subcategories include the delay discounting task and choice reaction time tasks, respectively. Theoretically, both of these tests should be affected by overvaluing of the US, but they could also be affected by temperament, in the sense that an impulsive individual may exhibit behavioral responses in these tasks. In this section, we will discuss how impulsivity is implied experimentally and describe how it appears that sign-trackers are more impulsive than goal-trackers.
Choice Impulsivity
In delay discounting, the subject must make one of two responses, one which results the delivery of a smaller immediate reward, while the other results in a delayed, albeit larger, reward. Either the amount of, or the delay to, the larger reward is varied, and from the subjects' responses an "indifference point" is calculated. The indifference point is the amount or delay at which the subject chooses the larger reward 50% of the time. Thus the larger this value, the less impulsive the subject is said to be (Bickel et al. 1999; de Wit and Richards 2004; Richards et al. 2012; Richards et al. 1997).
The relationship to sign-tracking in animal models of discounting is mixed, and may depend on genetic background. For example, the Lewis strain of rats have lower indifference points (Anderson and Woolverton 2005) and also learn to sign-track faster and to a larger degree compared to Fischer rats (Kearns et al. 2006). There is also some evidence from neurobiological studies that suggest that the mechanism underlying sign-tracking overlaps at least partially with that underlying impulsive choice. For example, lesions of the subthalamic nucleus decreased impulsive choice and impaired sign-tracking, and depletion of forebrain serotonin increased sign-tracking and the conditioned locomotor response to food (Winstanley et al. 2005; Winstanley et al. 2004). In another study, Long-Evans rats were tested in a PavCA paradigm and then tested under delay discounting conditions where the small reward was 1–2 pellets and the large reward was 5–10 pellets. The delays were 0, 10, 20, 40, and 60 seconds (Tomie et al. 1998). When tested in this manner, two subpopulations of rats emerged: "Sensitive" rats responded more for the large reward at the 0 and 10 second delays compared to "Insensitive" rats that were not affected by the delay and mostly chose the small reward. Interestingly, Sensitive rats sign-tracked 6 to 9-fold more than Insensitive rats, although it is not known if there was a difference in goal-tracking among these rats. Together these studies suggest a link between sign-tracking and choice impulsivity.
A study that explicitly compared sign- and goal-trackers assessed preference for a large (4 sucrose pellets) versus small (1 pellet) reward at 0, 3, 6,12, and 24 second delays (Lovic et al. 2011) provided contrasting results. Relative to goal-trackers, sign-trackers preferred the large reward at the 12 and 24 second delay, although the effect size was marginal. Additionally, in another study using rats selectively bred for locomotor response to novelty, sign-trackers were less impulsive in a delay discounting task, which is opposite to what one might hypothesize (Flagel et al. 2010). Yet, it is worth noting that some phenotypic differences in these lines may be a by-product of genetic drift. Nevertheless, in our own work using a large sample of heterogeneous stock rats, we have not observed a relationship between sign-tracking and delay discounting measures of impulsivity but have reported differences in action impulsivity (King et al. 2016).
Action Impulsivity
Another domain of impulsivity is action impulsivity, in which subjects fail to withhold a response at appropriate times. Several studies have demonstrated a link between sign-tracking and action impulsivity, as measured by tasks that require a response to be withheld in order for a reward to be delivered. For example, in the DRL (differential reinforcement of low rates of responding) task (Richards et al. 1993), subjects are only reinforced if they withhold a response for a predetermined period of time before responding. While there were no differences in overall responding or total reinforcers earned, sign-trackers, compared to goal-trackers, were less efficient in that they made more premature responses that did not result in reinforcement, which is consistent with a more impulsive phenotype (Lovic et al. 2011).
Another, more complicated test that measures impulsive action in addition to attention, is the serial reaction time test, also known as the choice reaction time task. In this task, rats must pay attention to multiple holes on a wall and enter the one that is illuminated very briefly with a small light in the hole. Correct responses are rewarded, while incorrect responses are not and result in a time-out period before the next trial. The task requires the subject to pay close attention so as not to miss which hole is illuminated. Thus, correct and incorrect responses can be measured and are thought to reflect deficits in attention (Robbins 2002). In addition, rats must refrain from entering the hole before the stimulus light appears, otherwise a time-out ensues and a premature response is recorded. These premature responses are the operational measure of impulsivity. In their study, Lovic et al. (2011) found that sign-trackers made more premature responses than goal-trackers but did not differ in the number of correct or incorrect responses. In our own work with Jerry Richards, we used a variant of this task in which rats must hold their snout in a center hole before responding and found a similar increase in premature responses (snout withdrawal) in sign-trackers compared to goal-trackers (King et al. 2016). We also did not observe any differences in correct or incorrect responses, nor did we see differences in reaction times, which are another measure of attention in this task. Together, these studies suggest that sign-tracking is associated with increased impulsive responding but not with attentional deficits. However, Martin Sarter and colleagues have reported attentional deficits in sign-trackers (discussed later) using more rigorous tests of attentional function (Koshy Cherian et al. 2017; Paolone et al. 2013; Pitchers et al. 2017).
In summary, most reports reveal that sign-trackers are more impulsive than goal-trackers. It may seem initially paradoxical that while sign-trackers are not different than goal-trackers during extinction of instrumental responses, there is a difference in instrumental responding when the rats must withhold responding. Why is this? First, during Pavlovian extinction, the CS is present, while during instrumental extinction there is no stimulus. Second, during the choice reaction time task, DRL, and devaluation tasks, while the Pavlovian CS is not present during the test trials, the unconditioned stimulus is. Thus, the presence of either the CS or the US is key in determining whether there are differences between sign- and goal-trackers. Indeed, when sign- and goal-trackers that had undergone instrumental extinction were presented with the CS anew, the food-seeking behavior of sign-trackers reinstated to a greater degree than goal-trackers (Yager and Robinson 2010).
Uncertainty and Suboptimal Choice
Because of the link between sign-tracking and impulsive behavior indicated earlier, others have suggested a link between sign-tracking and gambling-like behavior. A key feature of gambling scenarios is that rewards (i.e., money) are delivered intermittently and with varying magnitude. This is readily modeled in animals by replacing money with food as the reward. For example, in a set of studies, rats were presented with a CS that was followed by a US only half the time, and the number of sucrose pellets delivered varied such that one, two, or three pellets was delivered. This led to substantial increases in sign-tracking compared to the control group that got two pellets on 100% of the conditioning trials (Anselme et al. 2013; Davey et al. 1982; Robinson et al. 2014a; Robinson et al. 2015). Anselme (2016) points out that this cannot be accounted for by most theories of learning that rely on the CS-US contiguity as a key factor leading to increases in conditioned responding. Instead he argues that the magnitude and form (i.e., sign- versus goal-tracking) of the response are determined by motivational factors. To oversimplify his "incentive hope" model (Anselme this volume), rewards obtained during uncertain conditions make those rewards more motivationally relevant and valued, and thus conditioned responding is increased due to the reward's enhanced incentive salience. This is also consistent with work summarized by Hearst and Jenkins (Brown and Jenkins 1968; Hearst and Jenkins 1974) showing that manipulations that decrease the frequency of CS-US presentations (e.g., long intertrial intervals) also increase the magnitude and/or acquisition of sign-tracking.
One prediction based on this idea is that sign-trackers will make risky decisions in animal models of gambling-like behavior. One such model is the suboptimal choice procedure, in which rats must choose between two alternatives, one that leads to an infrequent reward that is preceded a by CS 100% of the time, the other that leads to a frequent reward that preceded by a CS half the time. Suboptimal choice, akin to gambling, is demonstrated when the rats choose the perfectly predicted but infrequent reward (Smith et al. 2016). A recent study tested the hypothesis that sign-trackers would be more sensitive to suboptimal choice (Lopez et al. 2018). First, sign- and goal-trackers were identified using standard techniques and then tested in the suboptimal choice procedure. The authors found that both sign- and goal-trackers exhibited a preference for the poorly predicted but frequent reward, thus behaving optimally. However, while these findings indicate that vulnerability to sign-tracking does not overlap with vulnerability to suboptimal choice, other studies suggest that incentive salience, as measured by sign-tracking behavior, is a mechanism that promotes suboptimal choice. Specifically, Chow et al. (2017) presented a lever (that could be sign-tracked) as the CS in one group and compared responding to a separate group where a light (that elicited only goal-tracking) was the CS. The lever promoted suboptimal choice behavior in this experiment, thus showing that a CS that has acquired incentive value can promote risky, gambling-like behavior. Interestingly, like Lopez et al. (2018), they did not observe an association between the magnitude of sign-tracking and choice behavior. While this may seem paradoxical, the mechanism underlying sign-tracking may not be the same as the predisposing factors that render an individual more likely to become a sign-tracker.
Discussion
What is particularly interesting is that, unlike PavCA, during delay discounting, omission, and the suboptimal choice task, the measures of action impulsivity occur during a period in which there is no stimulus immediately present. Therefore, the activation of the motivated response must be driven by (or poorly controlled by) an internal force, rather than being a conditioned response to an external stimulus. In other words, explanations for enhanced impulsivity in sign-trackers include (1) sign-trackers value the US more than goal-trackers and as a result are more likely to make premature responses, or (2) sign-trackers have poor inhibitory control despite valuing the US similarly compared to goal-tracking. One might expect that, if the US had enhanced value for sign-trackers, rats would have enhanced attention in tasks such as the choice reaction time task, but no study has demonstrated such an attentional enhancement. In fact, Paolone et al. (2013) demonstrated attentional deficits in sign-trackers trained in the sustained attention task, a cue-detection task that involves extended testing. Sign-trackers did poorly as this testing progressed, relative to goal-trackers, and fluctuated between periods of good and near-chance performance. Further, this poor attentional control was associated with lower levels of cortical acetylcholine levels. In a later paper (Koshy Cherian et al. 2017), they manipulated the acetylcholine system and demonstrated that experimental reductions of cortical acetylcholine increased the degree of sign-tracking. The authors concluded that this neurochemical deficit in sign-trackers causes a deficit in executive control over behavior, effectively unmasking motivated behaviors driven by CSs and/or USs (see Pitchers et al. 2017 for further discussion). This latter finding is somewhat at odds with other papers showing that nicotinic acetylcholine receptor agonists (such as nicotine itself) increase sign-tracking (Palmatier et al. 2013; Versaggi et al. 2016), although this is likely due to these manipulations affecting non-cortical areas of the brain. Regardless, these studies demonstrate that sign-tracking is associated with high levels of action impulsivity that are accompanied by poor attention, which, loosely speaking, could be conceptualized as another manifestation of poor self-regulation.
Thus far, we have reviewed findings that suggest sign-tracking reflects a trait associated with impulsivity, but that is also partially influenced by the current value of a given US. However, up until this point we have ignored drug USs, which are interesting for several reasons. First, drugs are a different class of US that are not present in the Pavlovian conditioned approach test at all. Second, sign-trackers prefer a cocaine US over a food US (Tunstall and Kearns 2015). Finally, the unconditioned vocalization response to cocaine is enormously different in sign- and goal trackers (Tripi et al. 2017). In the next section we will discuss these drug-induced vocalizations, how they are different in sign- and goal-trackers, and how they provide insight on the underlying nature of the differences between sign- and goal-trackers.
Ultrasonic Vocalizations
The correlated responses discussed in this section reveal several behavioral distinctions between sign- and goal-trackers, and other chapters in this volume suggest a strong link between sign-tracking, goal-tracking, and addiction-related behaviors (Levitch et al. this volume; Robinson et al. this volume; Tomie et al. this volume). However, all of the tasks previously described involve some form of learning, and there are few measures of unconditioned responsivity to rewards that have been thoroughly investigated. As such, this provides limited information regarding what predisposing factors lead to differences in sign- and goal-tracking. In the case of drugs of abuse, some have suggested that the locomotor response to reward presentation is a simple measure of the unconditioned response to rewards (Wise and Bozarth 1987). However, there are several dissociations between drug-induced locomotion and motivation, and studies have reported subtle differences or no differences in drug-induced locomotion between sign- and goal-trackers (Beckmann et al. 2011; Flagel et al. 2008; Tripi et al. 2017). Thus, the locomotor response to reward presentation may not necessarily reflect the motivational response to a given US. Alternatively, reward presentation evokes other unconditioned responses in rodents, including the production of ultrasonic vocalizations (USVs).
Relevance to Motivation
USVs are complex vocal responses produced by rodents occurring at a frequency above the upper limit of human hearing (> 20 kHz). Adult rats produce a repertoire of USVs that can be classified in two distinct categories based on their mean frequencies: 22 kHz (18–28 kHz) and 50 kHz calls (30–80 kHz) (Portfors 2007; Wohr and Schwarting 2013). Both of these categories of USVs have been proposed to serve a communicative function and may represent distinct motivational and affective states in response to different social and nonsocial situations (Knutson et al. 2002). Specifically, 22 kHz USVs are primarily produced in response to aversive contexts such as electric shock (van der Poel et al. 1989), intermale aggression (Sales 1972; Thomas et al. 1983), stress (Knapp and Pohorecky 1995), and drug withdrawal (Berger et al. 2013; Vivian and Miczek 1991). On the other hand, 50 kHz USVs predominate in positive contexts involving appetitive or reinforcing stimuli including mating (Burgdorf et al. 2008), play (Knutson et al. 1998), exploration (Blanchard et al. 1993) and also in response to abused drugs and their associated cues (Meyer et al. 2012). Thus, researchers in the field have proposed USVs as potential objective measures of negative and positive emotional states in preclinical models. Further, this measure has been suggested as a preclinical analogue to human "self-report" of subjective states that include both physiological and motivational changes in the organism (Brudzynski 2007; Mahler et al. 2013; Panksepp et al. 2002).
Importantly, the production of USVs can occur in both unconditioned and conditioned contexts. Unconditioned responding constitutes reflexive behaviors that occur naturally due to a given stimulus, and unconditioned USVs have been shown to be elicited by acute exposure to various manipulations, including those previously listed. USVs occurring prior to learning may reflect the initial subjective experience to a given stimulus. On the other hand, conditioned USV responding implies that some learning about the stimulus and its relationship to cues in the external and/or internal environment has occurred, and thus has come to elicit an affective response. Evidence for the production of conditioned USVs has been demonstrated by the ability of reward-paired cues alone to instigate vocalizations. For example, environments paired with drugs of abuse have been shown to produce USVs in the absence of the drug (Burgdorf et al. 2007; Knutson et al. 1998; Meyer et al. 2012), and 50 kHz USVs have been proposed to reflect anticipation of reward and reward-related cues (Brenes and Schwarting 2014; Ma et al. 2010).
Several rodent studies measuring both spontaneous and stimuli-induced USVs have confirmed stable individual differences in 50 kHz vocalizations, with some subjects exhibiting high USV rates and others exhibiting low USV rates (Ahrens et al. 2013; Mallo et al. 2007; Taracha et al. 2012). This bidirectional affective response, reflected by USV production, can be selectively bred for. Thus, natural variability of this measure can be used to study the neuroanatomical and pharmacological basis of individual differences in aspects of motivation and emotion (Brudzynski et al. 2011). In fact, according to Dickinson and Balleine (2002), the incentive value of rewards and their related stimuli are largely determined by the affective experience that results from consuming a reward. This perspective is supported by clinical studies in which positive responses to a drug during its initial use was associated with shorter latency for second use and increased willingness to take the drug in subsequent sessions (de Wit et al. 1986; Kollins et al. 2001; Volkow et al. 1999). Browning et al. (2011) demonstrated this relationship in rats by measuring cocaine-induced USVs. In this study, high rates of USVs on the first day of acquisition were positively correlated with the speed at which cocaine self-administration was acquired. Taken together, these studies provide evidence that assessing the individual differences in the initial subjective experience in response to rewards, particularly drug rewards, may reveal insight into future conditioned reward-direct behaviors.
Relationship to Sign- and Goal-Tracking
Previous work has assessed the relationship between incentive motivation and the production of conditioned USVs in response to both food and drug. In a study conducted by Brenes and Schwarting (2015), subjects learned to enter a runway maze to gain access to a food reward in a connected cage. In this, sign-tracking was characterized by repeated returns to the food-associated runway during the food access period and results indicated that subjects exhibiting high levels of sign-tracking also showed heightened conditioned 50 kHz USVs. They concluded that the reward-paired maze cue had been imbued with incentive salience by these individuals and its increased motivational value triggered appetitive USVs. In Tripi et al. (2017), we measured USV production in sign- and goal-trackers before, during, and after the presentation of a food-paired lever cues on the last day of PavCA (Day 5). Results of this study indicated only marginal differences in USV production at any point during the task, and this lack of differences may have been due to low cue-induced USVs in general. Although not specifically associated with cue or reward presentation, the only USV differences between sign- and goal-trackers occurred during the first 5 minutes of the task, indicating moderate differences in the conditioned affective response to reward-associated environments between these rats.
Differences between sign- and goal-trackers have also been observed in the conditioned and unconditioned USV response to drugs of abuse. For example, Meyer et al. (2012) measured USVs in a cocaine conditioned place preference (CPP) paradigm. They demonstrated that cocaine-treated sign-trackers produced more 50 kHz USVs compared to cocaine-treated goal-trackers for all cocaine-pairing sessions. Additionally, during the post-test (during which no drug was given), sign-trackers, unlike goal-trackers, exhibited preference for the cocaine-paired floor and also produced significantly more USVs than goal-trackers at this time. This finding was the first to suggest that sign-tracking individuals may have heightened sensitivity to the reinforcing effects of Pavlovian cocaine cues, and that the subjective value of the drug may facilitate this difference.
Differences in cocaine-induced USVs can also be observed in paradigms that do not require reward learning. We conducted a study in which sign- and goal-trackers were treated with 10 mg/kg (i.p.) cocaine and placed into a locomotor chamber (Tripi et al. 2017). On the first day of drug administration, sign-trackers produced significantly more 50 kHz USVs compared to goal-trackers, illustrating an inherent difference in the unconditioned affective response to cocaine. Notably, this response sensitized across repeated administration and also following a seven-day drug-free period in sign-trackers alone (see Figure 6.2). These findings substantiate a relationship between sign-tracking and cocaine-induced vocalizations, suggesting that the two may function through overlapping neural mechanisms. In fact, the more robust cocaine-induced USV rate observed in sign-trackers may reflect an increased sensitivity to the motivational properties of cocaine, and thus begin to explain previously reported variation between sign- and goal-trackers in drug seeking and taking behaviors.
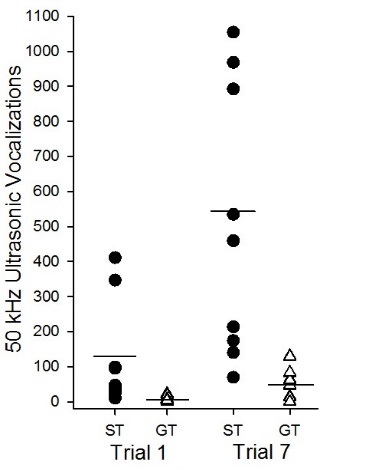
Figure 6.2: Sign-trackers emitted more acute and sensitized cocaine-induced USVs. Symbols indicate USVs by individual subjects on the first (Trial 1; ST mean=120, GT mean=4) and last (Trial 7; ST mean = 553, GT mean = 63) day of cocaine treatment.
Indexing Differences in the Subjective Response to Cocaine
The individual differences in cocaine-induced 50 kHz USVs between sign- and goal-trackers suggest different subjective responses to the drug unconditioned stimulus (US). Whereas it is unclear which aspects of cocaine's subjective effects are promoting this difference or what the mechanism is, there is some other evidence that informs these gaps in knowledge. Appetitive 50 kHz USVs in response to drugs of abuse, including cocaine, have been proposed to reflect positive hedonic states including euphoria (Burgdorf et al. 2011). Under this interpretation, sign-trackers would be more sensitive to pleasurable properties of cocaine, compared to goal-trackers. In this same vein, initial positive subjective responses to cocaine have been closely tied to subsequent drug-seeking and taking behaviors in both preclinical and clinical models. According to Barker et al. (2014) the presence or absence of a hedonic response to drug treatment may indicate differences in neural physiology that are responsible for differences in learning and even in the transition for drug use to dependence in humans. Thus, increased 50 kHz cocaine-induced USVs could serve as a predictor for these addiction-like behaviors. Interestingly, this interpretation fits well with previous findings regarding variation between sign- and goal-trackers in other cocaine paradigms. For example, although both sign- and goal-trackers will acquire cocaine self-administration at the same rate, when tested under progressive ratio, sign-trackers will work nearly twice as hard as goal-trackers for a single cocaine infusion (Saunders and Robinson 2011). This increased willingness to work could be facilitated by a more pronounced positive evaluation of the drug by sign-trackers during learning. In support of this, a study by Tunstall and Kearns (2015) similarly supports this notion of differential evaluation of cocaine. In this, subjects previously characterized and sign- and goal-trackers were trained to lever press for a food pellet and cocaine infusion. When given a choice to administer either, sign-trackers chose cocaine over food significantly more than goal-trackers. Further, Robinson et al. (2014b) suggested that tendency to sign-track may be reflective of an underlying trait to globally attribute incentive salience to reward stimuli, including interoceptive stimuli. Thus, sign-trackers may not only be more sensitive to the initial subjective effects of cocaine, but also may come to attribute incentive salience to the internal state associated with cocaine and thus be more likely to take and seek drug in the future.
Mechanisms of Individual Differences in Subjective Response to Cocaine
The individual variation in cocaine-induced USVs between sign- and goal-trackers may rely upon differences in the mesolimbic dopamine system, specifically in how rewards and their related cues are encoded. Previous reports have demonstrated that unlike goal-tracking, sign-tracking is dopamine-dependent and the acquired conditioned response can be blocked by DA antagonism (Flagel et al. 2011; Kuhn et al. this volume). Additionally, mesolimbic dopamine firing appears to be critical for the initiation of positive affect and the production of 50 kHz USVs (Hori et al. 2013). Thus, dopamine neurotransmission is a likely contributor to the differences in the affective response to cocaine among sign- and goal-trackers. For example, dopamine firing may be differentially affecting the initial reinforcing action of the drug and, with continued exposure, may affect variation in learning during drug conditioning. Importantly, it has been shown that cocaine-induced USVs sensitize in sign-trackers alone indicating unique alterations in the mesolimbic dopamine system through repeated cocaine administration (Burgdorf et al. 2001; Tripi et al. 2017; Willuhn et al. 2014).
The behavioral effects of cocaine are also mediated by other neurotransmitter systems that may play a role in the individual differences observed in sign- and goal-trackers. For example, the noradrenergic system has been shown to play a role in both sign-tracking and the production of stimulant-induced USVs. Activation of this system in the prefrontal cortex has been shown to increase during the pairing of a lever cue with a food reward (Tomie et al. 2004), suggesting that the increase has an underlying role in the development of the sign-tracking response. Additionally, antagonism of this system inhibits sign-tracking behavior in PavCA (Pasquariello et al. 2018). Similarly, stimulant-induced USVs are not completely abolished by DA antagonism and, additionally, hedonic responses in general may be DA independent (Berridge 2009; Wright et al. 2013), implying a contribution of additional neurochemical mechanisms. Wright et al. (2012) demonstrated that, along with dopamine, noradrenergic neurotransmission plays a critical role in stimulant-induced 50 kHz USVs. In fact, antagonism of the alpha-1 adrenergic receptor reliably attenuates 50 kHz USVs in rodents and has been reported to decrease the subjective effects of cocaine in humans (Newton et al. 2012).
Serving as a "self-report" of affective state, CS- and US-induced USVs may provide a reliable measure to index distinct aspects of reward sensitivity in rodent models. In fact, the robust relationship between sign-tracking and cocaine-induced 50 kHz USVs is particularly profound because of its implications as not only a predictive marker for sign-tracking and its correlated behaviors but also as an indicator of the underlying mechanisms that make sign- and goal-trackers distinct. The differential cocaine-induced USVs suggest that the sign- and goal-trackers differ in their initial subjective response to reward (whether that be the physical reward of the internal and external cues related to the reward), and this in turn may facilitate a divergence in reward-directed behavior in both food and drug paradigms.
Conclusion and the Potential Role of the US
In summary, the just discussed studies suggest that sign-tracking is persistent, albeit not entirely inflexible in the sense non-reinforcement can lead to a change in the topography of the response. This demonstrates that once the CS-US association is learned, the current value of the US can still influence the response to the CS in sign-trackers and goal-trackers. This does not seem to be due to enhanced motivation for the US, because there are no systematic differences between sign- and goal-trackers in tests that should be affected by the value of the US, including food self-administration. In fact, sign-trackers have attentional deficits, which is the opposite one might predict if they valued the US more than goal-trackers. Thus, especially considering that sign-trackers are more impulsive than goal-trackers in several paradigms, the most parsimonious explanation is that sign-tracking and goal-tracking reflect individual differences in a trait characterized by poor self-regulation that unmasks extreme incentive salience attribution to CSs. Still, because all of the tasks that involve assessing the value of the US involve learning, one cannot definitively conclude that sign- and goal-trackers do not differ in their response to USs.
A recent study by Patitucci et al. (2016) asserted that differences in sign- and goal-tracking arise "from the interaction between the palatability or value of the reinforcer and processes of association as opposed to dispositional differences (e.g., in sensory processes, 'temperament,' or response repertoire)". Their support for this assertion is that (1) in their experiments, the sign-tracking response to two different CSs, associated with either a food pellet or sucrose solution, were unrelated, and (2) the palatability of the reinforcer, as measured lick cluster size, was related to sign-tracking. These findings suggest that the US is initially more valued in sign-trackers than goal-trackers. There is some indirect evidence from other studies that sign-trackers may indeed value food and drug USs more. First, when sign- and goal-trackers self-administered food pellets on an FR1 schedule, sign-trackers did so at a faster rate, especially early in training (Yager and Robinson 2010), although the opposite effect was seen when a discriminative stimulus signaled food availability (Ahrens et al. 2016). Second, in rats selectively bred for high (HR) or low (LR) locomotor response to novelty, HR rats (who also exclusively sign-track) had more US-evoked dopamine compared to their goal-tracking LR counterparts, but there was no difference in outbred Sprague-Dawley rats (Flagel et al. 2011, see the session 1 US responses in Figures 2 and 3). Third, for cocaine, sign-trackers respond more in a PR test in the absence of cues (see Figure 4 in Saunders and Robinson 2011), and we found that one injection of cocaine induces many more ultrasonic vocalizations in sign-trackers compared to goal-trackers, and that this difference was further increased after repeated cocaine injections (Tripi et al. 2017). Finally, when considered in conjunction with the finding that larger reward magnitudes promote sign-tracking (e.g. Kasties et al. 2016), it seems there is ample evidence that the US initially has more incentive value in sign-trackers compared to goal-trackers, and this may explain why the CS acquires more incentive value in these subjects during Pavlovian paradigms. Data from action impulsivity studies are also consistent with this idea, because enhanced reinforcer value could explain increased impulsive responding. Surprisingly, there are no studies that test this idea directly. For example, a simple experiment would be to compare responding on a progressive ratio of reinforcement, which measure how hard subjects are willing to work for the reinforcer, in sign-trackers and goal-trackers. As it stands, a potential unconditioned response or behavioral "biomarker" that reliably predicts sign- and goal-tracking remains elusive.
References
- Ahrens, A. M., Nobile, C. W., Page, L. E., Maier, E. Y., Duvauchelle, C. L., & Schallert, T. (2013). Individual differences in the conditioned and unconditioned rat 50-kHz ultrasonic vocalizations elicited by repeated amphetamine exposure. Psychopharmacology (Berl) 229, 687–700.
- Ahrens, A. M., Singer, B. F., Fitzpatrick, C. J., Morrow, J. D., & Robinson, T. E. (2016). Rats that sign-track are resistant to Pavlovian but not instrumental extinction. Behavioural Brain Research 296, 418–430.
- Anderson, K. G., & Woolverton, W. L. (2005). Effects of clomipramine on self-control choice in Lewis and Fischer 344 rats. Pharmacology, Biochemistry, and Behavior 80, 387–393.
- Anselme, P. (2016). Motivational control of sign-tracking behaviour: A theoretical framework. Neuroscience and Biobehavioral Reviews 65, 1–20.
- Anselme, P. (this volume). Gambling hijacks an ancestral motivational system shaped by natural selection. In a. Tomie, & J. D. Morrow (Eds.), Sign-Tracking and Drug Addiction. Ann Arbor, MI: Maize Publishing.
- Anselme, P., Robinson, M. J., & Berridge, K. C. (2013). Reward uncertainty enhances incentive salience attribution as sign-tracking. Behavioral Brain Research 238, 53–61.
- Barker, D. J., Bercovicz, D., Servilio, L. C., Simmons, S. J., Ma, S., Root, D. H., Pawlak, A. P., & West, M. O. (2014). Rat ultrasonic vocalizations demonstrate that the motivation to contextually reinstate cocaine-seeking behavior does not necessarily involve a hedonic response. Addiction Biology 19, 781–790.
- Beckmann, J. S., & Chow, J. J. (2015). Isolating the incentive salience of reward-associated stimuli: Value, choice, and persistence. Learning & Memory 22, 116–127.
- Beckmann, J. S., Marusich, J. A., Gipson, C. D., & Bardo, M. T. (2011). Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behavioural Brain Research 216, 159–165.
- Berger, A. L., Williams, A. M., McGinnis, M. M., & Walker, B. M. (2013). Affective cue-induced escalation of alcohol self-administration and increased 22-kHz ultrasonic vocalizations during alcohol withdrawal: role of kappa-opioid receptors. Neuropsychopharmacology 38, 647–654.
- Berridge, K. C. (2009). "Liking" and "wanting" food rewards: Brain substrates and roles in eating disorders. Physiology Behavior 97, 537–550.
- Bickel, W. K., Odum, A. L., & Madden, G. J. (1999). Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology 146, 447–454.
- Blanchard, R. J., Yudko, E. B., Blanchard, D. C., & Taukulis, H. K. (1993). High-frequency (35–70 kHz) ultrasonic vocalizations in rats confronted with anesthetized conspecifics: Effects of gepirone, ethanol, and diazepam. Pharmacology Biochemistry and Behavior 44, 313–319.
- Breland, K., & Breland, M. (1961). The misbehavior of organisms. American Psychologist 16, 681–684.
- Brenes, J. C., & Schwarting, R. K. (2014). Attribution and expression of incentive salience are differentially signaled by ultrasonic vocalizations in rats. PLoS One 9, e102414.
- Brenes, J. C., & Schwarting, R. K. (2015). Individual differences in anticipatory activity to food rewards predict cue-induced appetitive 50-kHz calls in rats. Physiology and Behavior 149, 107–118.
- Brown, P. L., & Jenkins, H. M. (1968). Auto-shaping of the pigeon's key-peck. Journal of the Experimental Analysis of Behavior 11, 1–8.
- Browning, J. R., Browning, D. A., Maxwell, A. O., Dong, Y., Jansen, H. T., Panksepp, J., & Sorg, B. A. (2011). Positive affective vocalizations during cocaine and sucrose self-administration: A model for spontaneous drug desire in rats. Neuropharmacology 61, 268–275.
- Brudzynski, S. M. (2007). Ultrasonic calls of rats as indicator variables of negative or positive states: Acetylcholine-dopamine interaction and acoustic coding. Behavioral Brain Research 182, 261–273.
- Brudzynski, S. M., Silkstone, M., Komadoski, M., Scullion, K., Duffus, S., Burgdorf, J., Kroes, R. A., Moskal, J. R., & Panksepp, J. (2011). Effects of intraaccumbens amphetamine on production of 50 kHz vocalizations in three lines of selectively bred Long-Evans rats. Behavioural Brain Research 217, 32–40.
- Burgdorf, J., Knutson, B., Panksepp, J., & Shippenberg, T. S. (2001). Evaluation of rat ultrasonic vocalizations as predictors of the conditioned aversive effects of drugs. Psychopharmacology 155, 35–42.
- Burgdorf, J., Kroes, R. A., Moskal, J. R., Pfaus, J. G., Brudzynski, S. M., & Panksepp, J. (2008). Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. Journal of Comparative Psychology 122, 357–367.
- Burgdorf, J., Panksepp, J., & Moskal, J. R. (2011). Frequency-modulated 50 kHz ultrasonic vocalizations: a tool for uncovering the molecular substrates of positive affect. Neuroscience & Biobehavioral Reviews 35, 1831–1836.
- Burgdorf, J., Wood, P. L., Kroes, R. A., Moskal, J. R., & Panksepp, J. (2007). Neurobiology of 50-kHz ultrasonic vocalizations in rats: Electrode mapping, lesion, and pharmacology studies. Behavioural Brain Research 182, 274–283.
- Chang, S. E., & Smith, K. S. (2016). An omission procedure reorganizes the microstructure of sign-tracking while preserving incentive salience. Learning & Memory 23, 151–155.
- Chow, J. J., Smith, A. P., Wilson, A. G., Zentall, T. R., & Beckmann, J. S. (2017). Suboptimal choice in rats: Incentive salience attribution promotes maladaptive decision-making. Behavioural Brain Research 320, 244–254.
- Cleland, G. G., & Davey, G. C. L. (1982). The effects of satiation and reinforcer devaluation on signal-centered behavior in the rat. Learning and Motivation 13, 343–360.
- Colwill, R. M., & Motzkin, D. K. (1994). Encoding of the unconditioned stimulus in Pavlovian conditioning. Animal Learning & Behavior 22, 384–394.
- Davey, G. C., Oakley, D., & Cleland, G. G. (1981). Autoshaping in the rat: Effects of omission on the form of the response. Journal of the Experimental Analysis of Behavior 36, 75–91.
- Davey, G. C. L., & Cleland, G. G. (1984) Food anticipation and lever-directed activities in rats. Learning and Motivation 15, 12–36.
- Davey, G. C. L., Cleland, G. G., & Oakley, D. A. (1982). Applying Konorski's model of classical conditioning to signal-centered behavior in the rat: Some functional similarities between hunger CRs and sign-tracking. Animal Learning & Behavior 10, 257–262.
- De Tommaso, M., Mastropasqua, T., & Turatto, M. (2017). The salience of a reward cue can outlast reward devaluation. Behavioral Neuroscience 131, 226–234.
- de Wit, H., & Richards, J. B. (2004). Dual determinants of drug use in humans: Reward and impulsivity. Motivational Factors in the Etiology of Drug Abuse 50, 19–55.
- de Wit, H., Uhlenhuth, E. H., & Johanson, C. E. (1986). Individual differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug Alcohol Depend 16, 341–360.
- Derman, R., & Delamater, A. R. (2014). Conditioned sign tracking in rats is mediated by a representation of the goal. Society for Neuroscience Abstracts.
- Flagel, S. B., Clark, J. J., Robinson, T. E., Mayo, L., Czuj, A., Willuhn, I., Akers, C. A., Clinton, S. M., Phillips, P. E., & Akil, H. (2011). A selective role for dopamine in stimulus-reward learning. Nature 469, 53–57.
- Flagel, S. B., Robinson, T. E., Clark, J. J., Clinton, S. M., Watson, S. J., Seeman, P., Phillips, P. E., & Akil, H. (2010). An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: Implications for addiction. Neuropsychopharmacology: Official publication of the American College of Neuropsychopharmacology 35, 388–400.
- Flagel, S. B., Watson, S. J., Akil, H., & Robinson, T. E. (2008). Individual differences in the attribution of incentive salience to a reward-related cue: Influence on cocaine sensitization. Behavioural Brain Research 186, 48–56.
- Hearst, E., & Jenkins, H. M. (1974). Sign tracking: The stimulus-reinforcer relation and directed action. Proceedings of the Psychonomic Society, Austin, TX, pp. 1–49.
- Herrnstein, R. J., & Loveland, D. H. (1972). Food-avoidance in hungry pigeons, and other perplexities. Journal of the Experimental Analysis of Behavior 18, 369–383.
- Holland, P. C., Asem, J. S., Galvin, C. P., Keeney, C. H., Hsu, M., Miller, A., & Zhou, V. (2013). Blocking in autoshaped lever-pressing procedures with rats. Learning and Behavior, 42(1), 1–21.
- Holland P. C., & Straub, J. J. (1979). Differential effects of two ways of devaluing the unconditioned stimulus after Pavlovian appetitive conditioning. Journal of Experimental Psychology Animal Behavior Processes 5, 65–78.
- Hollis, K. (1982). Pavlovian conditioning of signal-centered action patterns and autonomic behavior: a biological analysis of function Advances in the study of behavior. Academic Press, Inc., pp 1–53.
- Hori, M., Shimoju, R., Tokunaga, R., Ohkubo, M., Miyabe, S., Ohnishi, J., Murakami, K., & Kurosawa, M. (2013). Tickling increases dopamine release in the nucleus accumbens and 50 kHz ultrasonic vocalizations in adolescent rats. Neuroreport 24, 241–245.
- Kasties, N., Starosta, S., Gunturkun, O., & Stuttgen, M. C. (2016). Neurons in the pigeon caudolateral nidopallium differentiate Pavlovian conditioned stimuli but not their associated reward value in a sign-tracking paradigm. Scientific Reports 6, 35469.
- Kearns, D. N., Gomez-Serrano, M. A., Weiss, S. J., & Riley, A. L. (2006). A comparison of Lewis and Fischer rat strains on autoshaping (sign-tracking), discrimination reversal learning and negative auto-maintenance. Behavioural Brain Research 169, 193–200.
- King, C. P., Palmer, A. A., Woods, L. C., Hawk, L. W., Richards, J. B., & Meyer, P. J. (2016). Premature responding is associated with approach to a food cue in male and female heterogeneous stock rats. Psychopharmacology, 233(13), 2593–2605.
- Knapp, D. J., & Pohorecky, L. A. (1995). An air-puff stimulus method for elicitation of ultrasonic vocalizations in rats. Journal of Neuroscience Methods 62, 1–5.
- Knutson, B., Burgdorf, J., & Panksepp, J. (1998). Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. Journal of Comparative Psychology 112, 65–73.
- Knutson, B., Burgdorf, J., & Panksepp, J. (2002). Ultrasonic vocalizations as indices of affective states in rats. Psychological Bulletin 128, 961–977.
- Kollins, S. H., MacDonald, E. K., & Rush, C. R. (2001). Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacology Biochemistry and Behavior 68, 611–27.
- Koshy Cherian, A., Kucinski, A., Pitchers, K., Yegla, B., Parikh, V., Kim, Y., Valuskova, P., Gurnani, S., Lindsley, C. W., Blakely, R. D., & Sarter, M. (2017). Unresponsive choline transporter as a trait neuromarker and a causal mediator of bottom-up attentional biases. The Journal of Neuroscience 37, 2947–2959.
- Kuhn, B. N., Campus, P., & Flagel, S. B. (this volume). The neurobiological mechanisms underlying sign-tracking behavior. In A. Tomie, & J. D. Morrow (Eds.), Sign-Tracking and Drug Addiction. Ann Arbor, MI: Maize Publishing.
- Levitch, E. A., Marcinkowski-Paulis, S. E., & Tomie, A. (this volume). Telling stories about sign-tracking boosts awareness of loss of self-control related to drug use. In A. Tomie, & J. D. Morrow (Eds.), Sign-tracking and drug addiction. Ann Arbor, MI: Maize Publishing.
- Locurto, C., Terrace, H. S., & Gibbon, J. (1976). Autoshaping, random control, and omission training in the rat. Journal of the Experimental Analysis of Behavior 26, 451–462.
- Lopez, P., Alba, R., & Orduna, V. (2018). Individual differences in incentive salience attribution are not related to suboptimal choice in rats. Behavioural Brain Research 341, 71–78.
- Lovic, V., Saunders, B. T., Yager, L. M., & Robinson, T. E. (2011). Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behavioural Brain Research 223, 255–261.
- Ma, S. T., Maier, E. Y., Ahrens, A. M., Schallert, T., & Duvauchelle, C. L. (2010). Repeated intravenous cocaine experience: development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behavioural Brain Research 212, 109–114.
- Mahler, S. V., Moorman, D. E., Feltenstein, M. W., Cox, B. M., Ogburn, K. B., Bachar, M., McGonigal, J. T., Ghee, S. M., & See, R. E. (2013). A rodent "self-report" measure of methamphetamine craving? Rat ultrasonic vocalizations during methamphetamine self-administration, extinction, and reinstatement. Behavioural Brain Research 236, 78–89.
- Mallo, T., Matrov, D., Herm, L., Koiv, K., Eller, M., Rinken, A., & Harro, J. (2007). Tickling-induced 50-kHz ultrasonic vocalization is individually stable and predicts behaviour in tests of anxiety and depression in rats. Behavioral Brain Research 184, 57–71.
- Meyer, P. J., Cogan, E. S., & Robinson, T. E. (2014). The form of a conditioned stimulus can influence the degree to which it acquires incentive motivational properties. PloS one 9, e98163.
- Meyer, P. J., Ma, S. T., & Robinson, T. E. (2012). A cocaine cue is more preferred and evokes more frequency-modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology 219, 999–1009.
- Moore, B. R. (1973). The role of directed Pavlovian reactions in simple instrumental learning in the pigeon Constraints on learning: Limitations and predispositions. Oxford, England: Academic Press, pp. xv, 488–xv, 488.
- Morrison, S. E., Bamkole, M. A., & Nicola, S. M. (2015). Sign tracking, but not goal tracking, is resistant to outcome devaluation. Frontiers in Neuroscience 9, 468.
- Morrow, J. D. (this volume). Relevance of sign-tracking to co-occurring psychiatric disorders. In A. Tomie, J. D. Morrow (Eds.), Sign-Tracking and Drug Addiction. Ann Arbor, MI: Maize Publishing.
- Nasser, H. M., Chen, Y-W., Fiscella, K., & Calu, D. J. (2015). Individual variability in behavioral flexibility predicts sign-tracking tendency. Frontiers in Behavioral Neuroscience, 9, 289.
- Newton, T. F., De La Garza, R., 2nd, Brown, G., Kosten, T. R., Mahoney, J. J., 3rd, & Haile, C. N. (2012). Noradrenergic alpha(1) receptor antagonist treatment attenuates positive subjective effects of cocaine in humans: a randomized trial. PLoS One 7: e30854.
- Palmatier, M. I., Marks, K. R., Jones, S. A., Freeman, K. S., Wissman, K. M., & Sheppard, A. B. (2013). The effect of nicotine on sign-tracking and goal-tracking in a Pavlovian conditioned approach paradigm in rats. Psychopharmacology (Berl) 226, 247–259.
- Panksepp, J., Knutson, B., & Burgdorf, J. (2002). The role of brain emotional systems in addictions: A neuro-evolutionary perspective and new "self-report" animal model. Addiction 97, 459–469.
- Paolone, G., Angelakos, C. C., Meyer, P. J., Robinson, T. E., & Sarter, M. (2013). Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. The Journal of neuroscience: the official journal of the Society for Neuroscience 33, 8321–8335.
- Pasquariello, K. Z., Han, M., Unal, C., & Meyer, P. J. (2018). Adrenergic manipulation inhibits Pavlovian conditioned approach behaviors. Behavioural Brain Research 339, 278–285.
- Patitucci, E., Nelson, A. J. D., Dwyer, D. M., & Honey, R. C. (2016). The origins of individual differences in how learning is expressed in rats: A general-process perspective. Journal of Experimental Psychology Animal Learning and Cognition 42, 313–324.
- Pitchers, K. K., Kane, L. F., Kim, Y., Robinson, T. E., & Sarter, M. (2017). "Hot" vs. "cold" behavioural-cognitive styles: Motivational-dopaminergic vs. cognitive-cholinergic processing of a Pavlovian cocaine cue in sign- and goal-tracking rats. European Journal of Neuroscience 46, 2768–2781.
- Portfors, C. V. (2007). Types and functions of ultrasonic vocalizations in laboratory rats and mice. Journal of American Association Laboratory Animal Science 46, 28–34.
- Richards, J. B., Gancarz, A. M., & Hawk, L. W. (2012). Animal models of behavioral processes that underlie the occurrence of impulsive behaviors in humans. In M. T. Bardo, D. H. Fishbein, & R. Milich (Eds.), Inhibitory Control and Drug Abuse Prevention: From Research to Translation (pp. 13–41). New York, NY; Dordrecht; London; Heidelberg: Springer.
- Richards, J. B., Mitchell, S. H., de Wit, H., & Seiden, L. S. (1997). Determination of discount functions in rats with an adjusting-amount procedure. Journal of the Experimental Analysis of Behavior 67, 353–366.
- Richards, J. B., Sabol, K. E., & Seiden, L. S. (1993). DRL interresponse-time distributions: Quantification by peak deviation analysis. Journal of Experimental Analysis of Behavior 60, 361–385.
- Robbins, T. (2002). The 5-choice serial reaction time task: Behavioural pharmacology and functional neurochemistry. Psychopharmacology 163, 362–380.
- Robinson, M. J., Anselme, P., Fischer, A. M., & Berridge, K. C. (2014a). Initial uncertainty in Pavlovian reward prediction persistently elevates incentive salience and extends sign-tracking to normally unattractive cues. Behavioural Brain Research 266C, 119–130.
- Robinson, M. J., Anselme, P., Suchomel, K., & Berridge, K. C. (2015). Amphetamine-induced sensitization and reward uncertainty similarly enhance incentive salience for conditioned cues. Behavioral Neuroscience 129, 502–511.
- Robinson, M. J., & Berridge, K. C. (2013). Instant transformation of learned repulsion into motivational "wanting". Current Biology 23, 282–289.
- Robinson, T. E., Carr, C., & Kawa, A. B. (this volume). The propensity to attribute incentive salience to drug cues and poor cognitive control combine to render sign-trackers susceptible to addiction. In A. Tomie, & J. D. Morrow (Eds.), Sign-Tracking and Drug Addiction. Ann Arbor, MI: Maize Publishing.
- Robinson, T. E., & Flagel, S. B. (2009). Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biological Psychiatry 65, 869–873.
- Robinson, T. E., Yager, L. M., Cogan, E. S., & Saunders, B. T. (2014b). On the motivational properties of reward cues: Individual differences. Neuropharmacology 76 Pt B, 450–459.
- Sales, G. D. (1972). Ultrasound and aggressive behaviour in rats and other small mammals. Animal Behavior 20, 88–100.
- Saunders, B. T., & Robinson, T. E. (2011). Individual variation in the motivational properties of cocaine. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology 36, 1668–1676.
- Smith, A. P., Bailey, A. R., Chow, J. J., Beckmann, J. S., & Zentall, T. R. (2016). Suboptimal choice in pigeons: Stimulus value predicts choice over frequencies. PloS one 11, e0159336.
- Stiers, M., & Silberberg, A. (1974). Lever-contact responses in rats: automaintenance with and without a negative response-reinforcer dependency. Journal of the Experimental Analysis of Behavior 22, 497–506.
- Stringfield, S. J., Boettiger, C. A., & Robinson, D. L. (2018). Nicotine-enhanced Pavlovian conditioned approach is resistant to omission of expected outcome. Behavioural Brain Research 343, 16–20.
- Taracha, E., Hamed, A., Krzascik, P., Lehner, M., Skorzewska, A., Plaznik, A., & Chrapusta, S. J. (2012). Inter-individual diversity and intra-individual stability of amphetamine-induced sensitization of frequency-modulated 50-kHz vocalization in Sprague-Dawley rats. Psychopharmacology (Berl) 222, 619–632.
- Thomas, D. A., Takahashi, L. K., & Barfield, R. J. (1983). Analysis of ultrasonic vocalizations emitted by intruders during aggressive encounters among rats (Rattus norvegicus). Journal of Comparative Psychology 97, 201–206.
- Tomie, A. (1996). Self-regulation and animal behavior. Psychological Inquiry 7, 83–85.
- Tomie, A., Aguado, A. S., Pohorecky, L. A., & Benjamin, D. (1998). Ethanol induces impulsive-like responding in a delay-of-reward operant choice procedure: impulsivity predicts autoshaping. Psychopharmacology 139, 376–382.
- Tomie, A., Jeffers, P., & Zito, B. (this volume). Sign-tracking model of the addiction blind spot. In A. Tomie, J. D. Morrow (Eds.), Sign-Tracking and Drug Addiction. Ann Arbor, MI: Maize Publishing.
- Tomie, A., Tirado, A. D., Yu, L., & Pohorecky, L. A. (2004). Pavlovian autoshaping procedures increase plasma corticosterone and levels of norepinephrine and serotonin in prefrontal cortex in rats. Behavioural Brain Research 153, 97–105.
- Tripi, J. A., Dent, M. L., & Meyer, P. J. (2017). Individual differences in food cue responsivity are associated with acute and repeated cocaine-induced vocalizations, but not cue-induced vocalizations. Psychopharmacology 234, 437–446.
- Tunstall, B. J., & Kearns, D. N. (2015). Sign-tracking predicts increased choice of cocaine over food in rats. Behavioural Brain Research 281, 222–228.
- van der Poel, A. M., Noach, E. J., & Miczek, K. A. (1989). Temporal patterning of ultrasonic distress calls in the adult rat: effects of morphine and benzodiazepines. Psychopharmacology (Berl) 97, 147–148.
- Versaggi, C. L., King, C. P., & Meyer, P. J. (2016). The tendency to sign-track predicts cue-induced reinstatement during nicotine self-administration, and is enhanced by nicotine but not ethanol. Psychopharmacology 233, 2985–2997.
- Vivian, J. A., & Miczek, K. A. (1991). Ultrasounds during morphine withdrawal in rats. Psychopharmacology (Berl) 104, 187–193.
- Volkow, N. D., Wang, G. J., Fowler, J. S., Logan, J., Gatley, S. J., Gifford, A., Hitzemann, R., Ding, Y. S., & Pappas, N. (1999). Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. American Journal of Psychiatry 156, 1440–1443.
- Williams, D. R., & Williams, H. (1969). Auto-maintenance in the pigeon: Sustained pecking despite contingent non-reinforcement. Journal of the Experimental Analysis of Behavior 12, 511–20.
- Willuhn, I., Tose, A., Wanat, M. J., Hart, A. S., Hollon, N. G., Phillips, P. E., Schwarting, R. K., & Wohr, M. (2014). Phasic dopamine release in the nucleus accumbens in response to pro-social 50 kHz ultrasonic vocalizations in rats. The Journal of neuroscience: The Official Journal of the Society for Neuroscience 34, 10616–10623.
- Winstanley, C. A., Baunez, C., Theobald, D. E., & Robbins, T. W. (2005). Lesions to the subthalamic nucleus decrease impulsive choice but impair autoshaping in rats: The importance of the basal ganglia in Pavlovian conditioning and impulse control. The European Journal of Neuroscience 21, 3107–3116.
- Winstanley, C. A., Theobald, D. E., Cardinal, R. N., & Robbins, T. W. (2004). Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. The Journal of neuroscience: The Official Journal of the Society for Neuroscience 24, 4718–4722.
- Wise, R. A., & Bozarth, M. A. (1987). A psychomotor stimulant theory of addiction. Psychological Review 94, 469–92.
- Wohr, M., & Schwarting, R. K. (2013). Affective communication in rodents: Ultrasonic vocalizations as a tool for research on emotion and motivation. Cell and Tissue Research 354, 81–97.
- Wright, J. M., Dobosiewicz, M. R., & Clarke, P. B. (2012). Alpha- and beta-Adrenergic receptors differentially modulate the emission of spontaneous and amphetamine-induced 50-kHz ultrasonic vocalizations in adult rats. Neuropsychopharmacology 37, 808–821.
- Wright, J. M., Dobosiewicz, M. R., & Clarke, P. B. (2013). The role of dopaminergic transmission through D1-like and D2-like receptors in amphetamine-induced rat ultrasonic vocalizations. Psychopharmacology (Berl) 225, 853–868.
- Yager, L. M., & Robinson, T. E. (2010). Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behavioural Brain Research 214, 30–34.
Notes
1. Here we use the term "cue" to refer to reward-related stimuli generally, while reserving "conditioned stimulus" (CS) for discussions involving Pavlovian conditioning.
2. It is worth mentioning the distinction between studies of sign-tracking versus sign-trackers (and goal-tracking/trackers). Sign-tracking refers to the degree of sign-tracking within a group, while sign-trackers refers to the subgroup of rats that preferentially sign-track or are predisposed to sign-track. This is important, because the purpose of an experiment differs depending on whether an entire group (i.e., including sign-trackers, goal-trackers, and intermediates) is being studied, or sign-trackers and goal-trackers are being explicitly compared. In the former case, the objective is to understand the brain and behavioral mechanisms that underlie sign-tracking, while in the latter case the objective is to determine the vulnerability factors that render particular individuals more likely to become sign-trackers and engage in other related behaviors.
3. However, another study found that an auditory CS did not block the sign-tracking response to a lever CS, suggesting that these different CSs engage different learning processes (Holland et al. 2013). While this does not rule out a role of the US, it does suggest that the lever CS is more prone to acquire incentive salience than auditory CSs (Meyer et al. 2014).
Sign Tracking Predicts Increased Chouce of Cocaine Over Food in Rat
Source: https://quod.lib.umich.edu/m/maize/mpub10215070/1:7/--sign-tracking-and-drug-addiction?rgn=div1;view=fulltext
0 Response to "Sign Tracking Predicts Increased Chouce of Cocaine Over Food in Rat"
Post a Comment